Ersetzen Hyaluronsäure Kapseln pharmazeutische Mittel?
Hyaluronsäure Kapseln sind besonders für ihre positive Auswirkung auf die Haut bekannt. Mittlerweile werden sie mit verschiedensten Zusatzstoffen angeboten und mit verschiedensten Werbeaussagen beworben. Der Hyaluronsäure selbst werden mehrere Wirkungsweisen nachgesagt, welche auch durch verschiedenste Studien belegt wurden.
Doch lassen sich mit den Kapseln die gleichen Ergebnisse erzielen? Und lassen sich mit der Einnahme von Hyaluronsäure Kapseln pharmazeutische Mittel ersetzen?
Was ist Hyaluronsäure?
Bei Hyaluron bzw. Hyaluronsäure handelt es sich um eine durchsichtige Flüssigkeit, die durch eine gelartige Konsistenz gekennzeichnet ist. Jene Substanz ist in der Haut des Menschen enthalten. Dort sorgt sie für Elastizität und Spannkraft. Mit fortschreitendem Alter nimmt der Gehalt von Hyaluron aber immer mehr ab.
Als Folge davon gibt die Spannkraft der Haut nach, sodass sie immer trockener wird. Mit der Zeit bilden sich dadurch immer tiefere Falten. Hyaluronsäure ist generell dadurch gekennzeichnet, dass sie große Mengen an Wasser binden kann. So ist ein Gramm der Substanz dazu imstande, bis zu sechs Liter Wasser zu binden.
Kommt die Säure mit Wasser in Berührung, erhält die Mischung eine gelartige Konsistenz. In dieser Form hat die Hyaluronsäure im Körper eine Vielzahl an Funktionen. So sorgt sie nicht nur für die Elastizität der Haut, sondern sie ist auch dafür verantwortlich, dass die Gelenke den täglichen Belastungen standhalten.
Dem Körper ist Hyaluron also bekannt. Aus diesem Grund gibt es bei der Einnahme dieser Substanz fast keine Nebenwirkungen. Ein Beispiel dafür sind Augentropfen, die über diesen Inhaltsstoff verfügen. Sie sind dazu imstande, die Augen zu beruhigen, wobei sie von fast allen Anwendern gut vertragen werden.
Wirkung von Hyaluronsäure Kapseln
Die Wirkungsweise von Hyaluron ist vielfältig und hängt beachtlich von der Größe der Moleküle ab. Dies gilt insbesondere dann, wenn die Säure in der Kosmetik zur Anwendung kommen soll. Generell wird hier zwischen zwei Größen unterschieden – der hochmolekularen und der niedermolekularen Hyaluronsäure.
Bei den meisten Herstellern werden beide Varianten einfach als Sodium Hyaluronate ausgewiesen. Wollen Anwender also genau wissen, welche Art von Hyaluronsäure sich im betreffenden Produkt befindet, sollten sie sich vom Hersteller genauere Informationen einholen.
Die hochmolekulare Hyaluronsäure ist dafür bekannt, dass sie nur kurzfristig wirkt. Sie ist daran zu erkennen, dass sich nach dem Auftragen ein feuchtigkeitsspendender Film auf der Haut bildet. Dabei hat diese Art der Hyaluronsäure eine entzündungshemmende Wirkung.
Leiden Sie also häufig an Infekten der Haut, sollten Sie zu jener Variante greifen. Kennzeichnend für solche Hyaluronsäure Kapseln ist eine Molekülmasse von 1500 kD und mehr. Die niedermolekulare Hyaluronsäure zeichnet sich durch eine Molekülmasse von 50 kD und weniger aus.
Sie wirkt langfristig, wobei Sie außerdem im Bindegewebe der Haut gespeichert wird. Sie verfügt daneben über eine nachhaltige Anti-Falten-Wirkung. Wollen Anwender dem Alterungsprozess entgegenwirken, sind Hyaluronsäure Kapseln auf niedermolekularer Basis die richtige Wahl.
Daneben stehen Ihnen Hyaluronsäure Kapseln auch mit der kleinsten Hyaluronsäure – der Oligohyaluronsäure – zur Verfügung. Sie verfügt über eine Molekülmasse von 3 kD und kleiner. Jene Substanz hat eine ähnliche Wirkung wie die niedermolekulare Variante.
Auch sie ist ein effektives Mittel gegen Falten und wird in Ihrem Bindegewebe gespeichert. Nutzer dürfen sich bei der Anwendung solcher Hyaluronsäure Kapseln über einen langfristigen Effekt freuen.
Wo wird Hyaluronsäure angewendet?
 Hyaluronsäure Kapseln kommen heute in den unterschiedlichsten Bereichen zur Anwendung. Denn im Körper des Menschen hat die Substanz einen wichtigen Stellenwert. Somit lässt sich mit diesem Medikament eine Vielzahl an Leiden behandeln.
Hyaluronsäure Kapseln kommen heute in den unterschiedlichsten Bereichen zur Anwendung. Denn im Körper des Menschen hat die Substanz einen wichtigen Stellenwert. Somit lässt sich mit diesem Medikament eine Vielzahl an Leiden behandeln.
Im kosmetischen Bereich
Bekanntheit erlangen Hyaluronsäure Kapseln vor allem im kosmetischen Bereich. Hier dienen sie dazu, das jugendliche Aussehen der Haut herzustellen. Auch haben sie eine feuchtigkeitsspendende Wirkung. Hyaluronsäure kann daneben auch direkt auf das Gesicht aufgetragen werden.
Für welche Art der Hyaluronsäure sich Anwender entscheiden, hängt vom gewünschten Effekt ab. Am wirkungsvollsten ist jedoch eine kombinierte Variante – also eine Mischung aus hochmolekularer und niedermolekularer Hyaluronsäure. So können Nutzer die Vorteile beider Ausführungen genießen.
Idealerweise macht die niedermolekulare Variante dabei den höchsten Anteil aus. Oligo-Hyaluron stellt auf dem Markt eine Neuheit dar, wobei der Kauf mit hohen Ausgaben verbunden ist.
In der Augenheilkunde
In der Augenheilkunde kommen Hyaluronsäure Kapseln zur Behandlung von Entzündungen zum Einsatz. Sind Ihre Augen häufig gerötet und gereizt, sollten sie die Einnahme jener Substanz in Erwägung ziehen.
In der Orthopädie
Hyaluronsäure Kapseln kommen in der Orthopädie zur Behandlung von Gelenksproblemen zur Anwendung. Denn die Säure dient im Körper unter anderem als Gelenksschmiere. Sie sorgt dafür, dass die Gliedmaßen selbst intensiven Belastungen stand halten.
Ist im Gelenk zu wenig Hyaluronsäure vorhanden, äußert sich das für die betroffene Person in großen Schmerzen. Der Gehalt an Hyaluronsäure lässt im Alter nach. Bemerkbar macht sich dies im Anfangsstadium durch knackende Gelenke. In solchen Fällen sollten Betroffene schnell handeln.
In der Orthopädie wird die Substanz aber nicht in Form von Hyaluronsäure Kapseln eingenommen – der Arzt spritzt die Flüssigkeit mithilfe einer Injektion direkt ins Gelenk des Betroffenen.
In der Urologie
Mittlerweile kommt Hyaluron auch zur Behandlung urologischer Beschwerden zum Einsatz. Zur Anwendung kommt die Substanz dabei vor allem zur Unterspritzung des vesikoureteralen Refluxes (VUR).
Ersetzen Hyaluronsäure Kapseln pharmazeutische Mittel?
Hyaluronsäure darf nicht als Allheilmittel angesehen werden. Sie können eine bestehende Therapie unterstützen, dürfen diese aber nicht ersetzen. Betroffene sollten sich bei Beschwerden daher immer an einen Arzt wenden. Nur er ist dazu imstande, eine genaue Diagnose zu stellen und die richtige Therapie zu verordnen.
Was sagt die Stiftung Warentest?
Stiftung Warentest gibt ihren Anwendern den Tipp, Hyaluronsäure Kapseln mit einem hohen Wirkstoffgehalt zu erstehen. Dabei gibt es zwischen Marken und Herstellern zum Teil große Unterschiede. Interessenten dürfen daher niemals zum erstbesten Produkt greifen.
Idealerweise informieren sie sich im Vorfeld genau über Verkäufer und die Zusammensetzung des Produkts. Außerdem sollten Anwender bedenken, dass die Wirkung der Hyaluronsäure Kapseln nach deren Absetzen wieder nachlässt. Es ist außerdem noch nicht möglich, Hyaluronsäure Kapseln gezielt für einen bestimmten Bereich anzuwenden.
Das gibt es bei Hyaluronsäure Kapseln zu beachten
Hyaluronsäure Kapseln sind in unterschiedlichen Preiskategorien zu haben. Abhängig sind die Kosten vom Gehalt an Hyaluron sowie vom Hersteller. Greifen Sie niemals zum erstbesten Produkt, wenn Sie Geld sparen wollen. Vergleichen Sie im Vorfeld am besten immer die Ware mehrerer Anbieter miteinander.
Doch machen Sie den Preis nicht zum alleinigen Entscheidungskriterium. Viel wichtiger ist es, dass die Qualität der Hyaluronsäure Kapseln stimmt. Werfen Sie deshalb einen Blick auf die Inhaltsstoffe und lassen Sie sich vom Hersteller Informationen über den Gehalt und die Art vom Hyaluron zukommen.
Denn auch von diesem Faktor werden Ihre laufenden Kosten beeinflusst. Wollen Sie den besten Effekt erzielen, sollten Sie pro Tag zwischen 200 und 300 Milligramm Hyaluron einnehmen. Einige Hyaluronsäure Kapseln enthalten nur 50 Milligramm an Hyaluron, während sich in einigen Produkte mehr als das Doppelte befindet.
Und je höher das Hyaluron dosiert ist, desto seltener müssen Sie die Hyaluronsäure Kapseln nachkaufen.
Wie viel mg Hyaluronsäure benötigt der Körper pro Tag?
Pro Tag ist eine Dosis zwischen 200 und 300 Milligramm empfehlenswert. Sie können Hyaluronsäure Kapseln, soweit ihr Anteil an Hyaluron diesen Wert nicht übersteigt, täglich einnehmen. Hyaluronsäure Kapseln sind gut verträglich, wobei bisher keine Nebenwirkungen bekannt sind. Im Zweifelsfall können Sie sich vor der Einnahme mit einem Arzt absprechen.
Wie lange kann bzw. sollte man Hyaluronsäure Kapseln einnehmen?
 Hyaluronsäure Kapseln sind für den langfristigen Gebrauch gedacht. Denn eine kurze Einnahme hat zumeist keinen Effekt. Oft müssen Betroffene die Präparate daher über mehrere Monate hinweg einnehmen. Denn es braucht einiges an Zeit, bis die Speicher der Zellen wieder gefüllt sind.
Hyaluronsäure Kapseln sind für den langfristigen Gebrauch gedacht. Denn eine kurze Einnahme hat zumeist keinen Effekt. Oft müssen Betroffene die Präparate daher über mehrere Monate hinweg einnehmen. Denn es braucht einiges an Zeit, bis die Speicher der Zellen wieder gefüllt sind.
Nach dem Absetzen der Hyaluronsäure Kapseln nimmt der Hyalurongehalt im Organismus wieder ab. Sie müssen dann damit rechnen, dass Ihre Beschwerden wieder auftreten und die Falten tiefer werden.
Haben die Präparate Nebenwirkungen?
Die Nebenwirkungen der Hyaluronsäure selbst sind keine bekannt. Da die Substanz vom Körper hergestellt wird, ist sie gut verträglich. Trotzdem sollten Sie vor dem Kauf einen Blick auf die Zusammensetzung der Hyaluronsäure Kapseln werfen. So stellen Sie sicher, dass Sie auf keinen der zusätzlichen Inhaltsstoffe allergisch sind. Kontaktieren Sie im Zweifelsfall immer den Hersteller.
Alternativen zu Hyaluronsäure Kapseln
Eine beliebte Alternative zu Hyaluron Kapseln sind Cremes. Dabei wird das Hyaluron direkt auf das Gesicht und damit den gewünschten Wirkbereich aufgetragen. Erfolgt dies regelmäßig, gewinnt Ihre Haut an Elastizität. Sie wirken dadurch jünger und frischer.
Auch Cremes sind generell gut verträglich. In ihnen ist des Weiteren sowohl nieder- als auch hochmolekulares Hyaluron enthalten. Sie dringen damit selbst in die tiefer liegenden Hautschichten ein. Doch genauso wie bei den Hyaluronsäure Kapseln wird das über die Haut zugeführte Hyaluron nach einiger Zeit vom Körper abgebaut.
Wollen Sie einen dauerhaften Effekt sehen, müssen Sie die Creme langfristig anwenden. Eine weitere Alternative zu Kapseln ist das Hyaluron Gel. Es wird – genauso wie die Creme – auf Gesicht und Dekolleté aufgetragen. Zu empfehlen ist Ihnen die Behandlung mit Gel vor allem dann, wenn die betreffende Partie schon stark erschlafft ist.
Denn jenes Produkt verjüngt nicht nur das Hautbild, sondern versorgt Sie auch mit Feuchtigkeit und wichtigen Mineral- und Inhaltsstoffen.
Fazit
Hyaluronsäure ist eine Substanz, die vom Körper selbst hergestellt wird. Darum gelten Hyaluronsäure Kapseln als gut verträglich. Mittlerweile kommen sie in den unterschiedlichsten Sparten zur Anwendung. So lassen sich mit ihnen unter anderem Gelenksbeschwerden, gereizte Augen oder trockene Haut behandeln.
Allerdings müssen Anwender im Vorfeld sicherstellen, dass es sich bei den betreffenden Hyaluronsäure Kapseln um hochwertige Erzeugnisse handelt. Auch kann die Einnahme solcher Substanzen niemals den Arztberuf ersetzen. Dies gilt insbesondere dann, wenn es sich um schmerzhafte Leiden wie Gelenksprobleme und Infekte handelt.
In diesem Fall ist es wichtig, sich eine ärztliche Diagnose einzuholen. Nur so lassen sich ernste Grundleiden ausschließen.
Wie wirkt pharmazeutisches Kollagen?
Stress und Umweltverschmutzung führen dazu, dass immer Menschen Probleme mit ihrer Haut oder ihrem Darm haben. Sowohl in Fällen von vorzeitiger oder zu rascher Hautalterung, als auch bei chronischen Darmentzündungen wird häufig pharmazeutisches Kollagen empfohlen.
Doch ist es sinnvoll? Um was genau handelt es sich eigentlich und weshalb ist es pharmazeutisch?
Was ist Kollagen?
Kollagene sind aus Eiweiß bestehende Faserbündel, die vor allem in den Bindegeweben des menschlichen und auch tierischen Körpers vorkommen. Das bedeutet, dass sie beim Aufbau von Sehnen, Bändern, Knochen, Knorpeln und vor allem auch der Haut eine wichtige Rolle spielen. Sie werden von allen vielzelligen Tieren selbst im Körper produziert.
Als pharmazeutisch werden sie dann bezeichnet, wenn sie in pharmazeutischen Produkten, also in Arzneimitteln, eingesetzt werden. Dabei werden sie nicht nur als Wirkstoff, sondern auch zur Umhüllung von Medikamenten, in blutstillenden Schwämmchen und als Blutplasmaersatz eingesetzt. Kollagene sind als natürlicher Bestandteil des Körpers für den Menschen auch in großen Mengen gut verträglich.
Wie wirkt das pharmazeutische Mittel?
Mit zunehmendem Alter lässt die Kollagenproduktion im Körper nach. Gleichzeitig führen Stress und Umweltbelastungen dazu, dass der Bedarf des Körpers steigt. Dann kann ihm über ein pharmazeutisch hergestelltes Mittel zusätzliches Kollagen zugeführt werden, um den Bedarf zu decken und einem Mangel entgegenzuwirken.
Wirkung auf die Haut
Die Kollagenfasern in der Haut binden Wasser und sorgen dadurch für ausreichende Feuchtigkeit der Haut. Sie wird gestrafft und ist elastischer. Ein Mangel an Kollagen in der Haut führt zu Austrocknung und Erschlaffung. So entstehen die gefürchteten Falten, die uns auf andere Menschen alt wirken lassen.
Pharmazeutische Kollagenpreparate sollen die für die Erneuerung der Haut notwendigen Kollagene liefern und so in Kombination mit Wasser die Widerherstellung der Spannkraft und Linderung der Falten ermöglichen.
Wirkung auf die Darmgesundheit
Im Darm wirkt Kollagen beruhigend auf die Schleimhäute. Es unterstützt die Reparatur von beschädigten Zellwänden und versiegelt dadurch die schützende Schleimhautschicht, die den ganzen Magen-Darm Trakt überzieht. Aus diesem Grund werden pharmazeutische Kollagenpreparate häufig bei entzündlichen Erkrankungen des Verdauungstraktes eingesetzt.
Insbesondere bei chronischen Darmerkrankungen sollen sie helfen den erhöhten Kollagenbedarf des Körpers auszugleichen.
Ist die Wirksamkeit von Kollagen bewiesen?
Obwohl schon viele Studien zur Wirkung von Kollagen auf die Haut durchgeführt wurden, steht ein wissenschaftlich anerkannter Nachweis der Wirksamkeit von pharmazeutischen und kosmetischen Mitteln noch aus. Die bisherigen Studienergebnisse deuten zwar auf eine positive Wirkung hin, werden aber allgemein als nicht aussagekräftig genug beurteilt.
Die Wirksamkeit des körpereigenen Kollagens gilt allerdings als erwiesen und die pharmazeutischen Kollagene sind unschädlich für den Körper.
In welchen Formen ist Kollagen erhältlich?
 Pharmazeutisches Kollagen ist sowohl zur äußerlichen Anwendung, in der Form von Cremes, als auch zur innerlichen Anwendung, in der Form von Nahrungsergänzungsmitteln, erhältlich. Welche Form Sie wählen sollten, hängt nicht nur von Ihrer bevorzugten Anwendungsform ab. Sie sollten auch das Ziel der Behandlung bedenken.
Pharmazeutisches Kollagen ist sowohl zur äußerlichen Anwendung, in der Form von Cremes, als auch zur innerlichen Anwendung, in der Form von Nahrungsergänzungsmitteln, erhältlich. Welche Form Sie wählen sollten, hängt nicht nur von Ihrer bevorzugten Anwendungsform ab. Sie sollten auch das Ziel der Behandlung bedenken.
Anwendung in Cremes
Cremes werden direkt auf die Haut aufgetragen. Sie ziehen rasch ein und entfalten daher auch schnell ihre Wirkung. Zumeist spenden sie gleichzeitig mit dem Kollagen auch Feuchtigkeit, um den durch den Kollagenmangel entstandenen Wasserverlust auszugleichen.
Sie haben den Vorteil, dass sie gezielt auf eine bestimmte Hautpartie aufgetragen werden können, um ihre Wirkung genau am gewünschten Ort zu entfalten. Da sie nicht eingenommen werden, haben Sie jedoch keine Wirkung auf den Darm, die Knochen oder Knorpel.
Auch gehen die so gespendeten Kollagene rasch wieder verloren, was eine häufige Anwendung erforderlich macht.
Anwendung als Nahrungsergänzungsmittel
In der Form von Nahrungsergänzungsmitteln werden Kollagene oral eingenommen. Dadurch gelangen sie direkt in den Magen-Darm Trakt und können ihre heilende Wirkung dort direkt entfalten. Für eine gezielte Behandlung der Haut, kann die Aufnahme über Nahrungsergänzungsmittel jedoch von Nachteil sein, da nur ein geringer Teil der Kollagene tatsächlich am gewünschten Wirkungsort ankommt.
Die Aufnahme über den Verdauungstrakt führt dazu, dass auch die Kollagene verdaut werden. Das bedeutet sie werden in ihre Bestandteile, verschiedene Aminosäuren, zerlegt und müssen danach erst wieder neu gebildet werden. So erhält der Körper zwar die nötigen Bausteine, aber keine fertigen Kollagene.
Kapseln: Kapseln sind vor allem schnell und einfach zu schlucken. Sie tragen den Wirkstoff bis in den Magen und werden dort nur langsam verdaut was zu einer allmählicheren Freisetzung führt. Allerdings tun sich viele Menschen mit dem Schlucken von Kapseln schwer und ihre Verdauung ist nicht einfach für den Magen.
Wenn Ihr Magen bereits durch eine Entzündung geschwächt ist, ist es besser ihn nicht noch zusätzlich zu belasten. Wählen sie dann also lieber eine andere Einnahmeform.
Pulver: Kollagenpulver ist fast gänzlich geschmacksneutral. Es kann also bequem in Wasser aufgelöst und getrunken werden. Wenn Ihnen das zu langweilig schmeckt, können Sie durchaus auch ein anderes Getränk wählen, ohne die Wirkung des Kollagens zu beeinträchtigen.
So ist zum Beispiel die Aufnahme mit einem Milkshake sehr beliebt. Bedenken Sie aber auch dabei, dass Sie einen kranken Verdauungstrakt nicht weiter belasten sollten, und wählen Sie ein möglichst gut verträgliches Getränk.
Trinkampullen: Trinkampullen können besonders schnell und einfach eingenommen werden und auch die Aufnahme des Kollagens erfolgt bei dieser Einnahmeform am schnellsten. Sie werden in unterschiedlichen Geschmacksrichtungen verkauft.
Es ist jedoch ratsam bei der Auswahl vor allem auf zusätzliche Inhaltsstoffe und ihre Wirkung zu achten. Die Trinkampullen sind etwas teurer als die anderen Formen und nehmen bei der Mitnahme in die Arbeit mehr Platz in der Tasche ein.
Fazit
Pharmazeutisches Kollagen soll sowohl bei der Bekämpfung der Hautalterung als auch bei der Widerherstellung der Darmschleimhaut bei Erkrankungen des Verdauungstraktes helfen. Seine Wirksamkeit gilt jedoch nicht als erwiesen. Gegen Falten gelten Hautcremes und gegen Darmerkrankungen Kollagen Nahrungsergänzungsmittel als die beste Anwendungsform.
Chemische Peelings – Wie gefährlich ist das pharmazeutische Mittel wirklich?
Mit einem chemischen Peeling wird das Hautbild und der Teint in kurzer Zeit perfektioniert und erste Fältchen werden reduziert. Außerdem werden Hautverfärbungen, wie zum Beispiel Pigmentflecken, ausgeglichen. Zudem kommt es zu einer Verminderung von vielen Hautproblemen.
Bei einem chemischen Peeling werden säurehaltige Produkte eingesetzt. Die Anwendung von chemischen Peelings erfolgt im Gesicht, Dekollete- sowie Halsbereich. Möglich, aber selten sind auch Behandlungen an den Armen, am Rücken und an den Händen.
Was sind chemische Peelings?
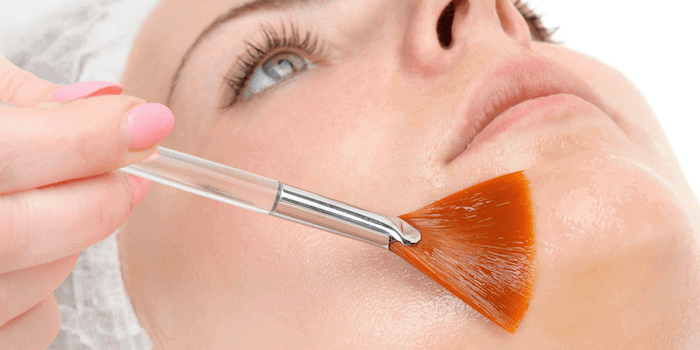 Bei einem chemischen Peeling handelt es sich um eine Behandlung, bei welcher die beschädigten Hautaußenschichten mit einer Säurelösung beseitigt werden. Die Behandlung ist pharmazeutisch. Die Hautstruktur wird durch das chemische Peeling verbessert und geglättet. Es ist wirksam bei Falten, Gesichtsunreinheiten sowie Hautpigmentierung.
Bei einem chemischen Peeling handelt es sich um eine Behandlung, bei welcher die beschädigten Hautaußenschichten mit einer Säurelösung beseitigt werden. Die Behandlung ist pharmazeutisch. Die Hautstruktur wird durch das chemische Peeling verbessert und geglättet. Es ist wirksam bei Falten, Gesichtsunreinheiten sowie Hautpigmentierung.
Es handelt sich um eine nichtinvasive Behandlungsmethode, die zu einer Verjüngung sowie Verbesserung der Haut führt. Besonders geeignet sind die chemischen Peelings zur Korrektur von Narben, Glättung sowie Straffung der oberen Hautschicht, Verbesserung des Hautbildes, Entfernung von Verfärbungen und Falten.
Welche Arten von chemischen Peelings gibt es?
Es gibt unterschiedliche Produkte, die für den jeweiligen Hauttyp geeignet sind. Die bekanntesten chemischen Peelings sind auf Basis von Alpha- Hydroxysäure (AHA), auf Basis von Beta-Hydroxysäure (BHA) sowie auf Basis von Poly-Hydroxysäure (PHA).
Alpha-Hydroxysäure (AHA)
Zu den chemischen Peelings auf Basis von Alpha-Hydroxysäure zählen die Peelings mit Fruchtsäure, Glykolsäure, Mandelsäure und Milchsäure. Alpha-Hydroxysäure wirkt glättend auf der Haut, regt die Produktion von Kollagen an, steigert das Feuchtigkeitsbindungsvermögen der Haut, vermindert Aknennarben sowie Verfärbungen.
Fruchtsäure: Peelings mit Fruchtsäure wirken an der oberen Hautschicht und sind geeignet bei Akne, bei Lichtschädigung und Faltenbildung der Haut. Außerdem können Pigmentveränderung mit Fruchtsäure sehr gut behandet werden.
Glykolsäure: Glykolsäure dringt tief in die Haut ein und stimuliert die Kollagenproduktion. Außerdem ist Glykolsäure ein sehr guter Feuchtigkeitsspender. Geeignet ist Glykolsäure bei sonnengeschädigter Haut, bei schuppiger Haut, feuchtigkeitsarmer Haut sowie bei geschlossenen Mitessern.
Mandelsäure: Mandelsäure dringt nicht so tief in die Haut ein. Mandelsäure ist entzündungshemmend und sehr gut verträglich. Geeignet ist sie zur Anwendung bei feuchtigkeitsarmer Haut.
Milchsäure: Die Milchsäure ist ein Bestandteil der Haut. Sie erhöht den Feuchtigkeitsgehalt sowie den Gehalt an Ceramiden. Geeignet ist die Milchsäure bei feuchtigkeitsarmer Haut und bei empfindlicher Haut.
Beta-Hydroxysäure (BHA)
Beta-Hydroxysäure ist fettlöslich. Sie wirkt in den Poren und befreit diese von Verstopfungen, ist gut verträglich, hat eine entzündungshemmende Eigenschaft, wirkt gut gegen Mitesser sowie Unreinheiten und ist für empfindliche Haut geeignet.
Salicylsäure: Salicylsäure ist entzündungshemmend und reduziert Besiedelung durch Haarbalgmilben. Geeignet ist sie bei Mitessern sowie bei öliger Haut. Bei Unverträglichkeit gegen Acetylsalicylsäure ist sie nicht geeignet.
Polyhydroxy Acids (PHA)
Zu den Peelings auf Polyhydroxy Acids-Basis zählen Gluconolactone und Lactobionsäure. Sie wirken antioxidativ, entzündungshemmend sowie antibakteriell. Sie dringen nicht so tief in die Haut vor und sind mild.
Gluconolactone : Gluconolactone wirkt antioxidativ. Nach der Behandlung mit Gluconolactone kommt es zu keiner erhöhten Lichtempfindlichkeit. Gluconolactone erhält zudem die Elastizität der Haut.
Lactobionsäure: Lactobionsäure ist mild und antioxidativ. Sie ist ein sehr guter Feuchtigkeitsspender. Geeignet ist sie bei sehr empfindlicher Haut, bei feuchtigkeitsarmer Haut, schuppiger und sonnengeschädigter Haut.
Eignen sich chemische Peelings für die Anwendung zu Hause?
 Normalerweise werden chemische Peelings von medizinischen Kosmetikern, von Dermatologen und vom Fachpersonal in Kliniken durchgeführt. Chemische Peelings eignen sich jedoch auch für die Anwendung zu Hause, vorausgesetzt man befolgt wichtige Regeln zur Anwendung. Meistens werden zu Hause Präparate auf Basis von Alpha-Hydroxysäure verwendet.
Normalerweise werden chemische Peelings von medizinischen Kosmetikern, von Dermatologen und vom Fachpersonal in Kliniken durchgeführt. Chemische Peelings eignen sich jedoch auch für die Anwendung zu Hause, vorausgesetzt man befolgt wichtige Regeln zur Anwendung. Meistens werden zu Hause Präparate auf Basis von Alpha-Hydroxysäure verwendet.
Die Anwendung von chemischen Peelings ist sehr einfach. Am wichtigsten ist es, dass man zunächst das Gesicht gründlich reinigt. Nach der Reinigung kann das Peeling verwendet werden. Chemische Peelings werden nicht abgespült. In diesem Punkt unterscheiden sie sich von den üblichen Peelings.
Nach dem Peeling können Feuchtigkeitspflegeprodukte eingesetzt werden. Die Haut ist nach dem chemischen Peeling sehr empfindlich. Deshalb ist es wichtig, Pflegeprodukte mit einem hohen Lichtschutzfaktor zu verwenden. Empfehlenswert ist es, das Peeling über Nacht einwirken zu lassen.
Welche Komplikationen und Risiken können bei pharmazeutischen Behandlungen auftreten?
Bei chemischen Peelings kommt es sehr selten zu Komplikationen, da die Behandlung pharmazeutisch ist. Zu den Risiken zählen Infektionen, Narben, Schwellungen, Veränderungen des Hauttons sowie Ausbrüche von Fieberbläschen. Die Risiken können vermindert werden, indem die Anweisungen des Arztes vollkommen befolgt werden.
Die Nebenwirkungen bei milden chemischen Peelings sind Stechen, Rötungen sowie Krustenbildung, die in wenigen Tagen nach der Behandlung nachlassen. Ausgeprägter sind die Nebenwirkungen bei tiefen chemischen Peelings, bei welchen die Erholungszeit länger dauert.
Für wen ist die Behandlung mit einem chemischen Peeling nicht geeignet?
Für Personen, die unter empfindlicher Haut, unter Hautentzündungen oder unter erweiterten Äderchen im Wangen- und Nasenbereich leiden, ist ein chemisches Peeling nicht so gut geeignet. Man sollte in diesen Fällen mit einem Dermatologen klären, ob ein chemisches Peeling zu empfehlen ist.
Zudem sind chemische Peelings während der Schwangerschaft und der Stillzeit nicht empfehlenswert. Wenn bestimmte Medikamente, wie zum Beispiel Präparate mit Vitamin A oder Antibiotika, eingenommen werden, sollte man ebenfalls auf das chemische Peeling verzichten.
Fazit
Chemische Peelings sind nicht gefährlich. Die Behandlung ist pharmazeutisch. Durch sie wird das Hautbild deutlich verbessert. Es ist empfehlenswert, mit einem milden chemischen Peeling anzufangen. Die tägliche Anwendung ist nur dann sinnvoll, wenn die Haut dies auch verträgt.
Chemische Peelings sollten abends durchgeführt werden, damit sich die Haut über Nacht erholen kann. Zudem ist es wichtig, dass die Säure nach dem persönlichen Hauttyp ausgewählt wird, um ein bestmögliches Ergebnis zu erzielen.
Wie sinnvoll sind pharmazeutische Anti Aging Cremes?
Anti Aging Cremes sind in jeder Drogerie oder im Supermarkt erhältlich. Aber worauf sollte beim Kauf des Pflegeproduktes geachtet werden? Um eine erfolgreiche Wirkung zu erzielen, spielen vor allem die Inhaltsstoffe eine große Rolle.
Bei einer hochwertigen Creme sollten neben Hyaluron auch viele Aminosäuren vorhanden sein. Die Creme muss auf die Bedürfnisse von reifer Haut abgestimmt sein. Neben einer feuchtigkeitsspendenden Wirkung sollten Anti Aging Cremes auch die Elastizität der Haut verbessern.
Was sind pharmazeutische Anti Aging Cremes?
Die Haut wird mit zunehmendem Alter immer sensibler und dünner. Die Elastizität nimmt ab und an unbedeckten Hautstellen treten Falten und Pigmentflecken auf. Pharmazeutische Anti Aging Cremes sind optimal dafür geeignet, um mit wirkungsvollen Inhaltsstoffen gegen Falten anzukämpfen. Die Cremes sind in der Regel mit vielen Vitaminen, pflanzlichen Ölen und feuchtigkeitsspendenden Inhaltsstoffen ausgestattet.
Unter anderem wird für die Pflege hochkonzentriertes Aloe Vera eingesetzt. Bei einigen Anti Aging Produkten wird ein besonderer Wirkstoff verwendet, sodass die Haut wie bei einer Sonnencreme mit einem UV-Lichtschutz gegen gefährliche Sonnenstrahlen geschützt wird.
Wie wirken die Cremes?
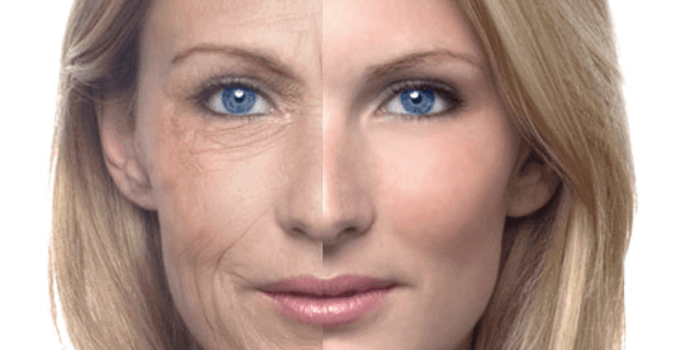 Die Konsistenz einer Anti Aging Creme ist besonders reichhaltig. Sie riecht frisch und zieht in den meisten Fällen ohne Rückstände ein. In den Anti Aging Cremes sind keine Silikone, Parabene oder PEGs vorhanden. Anti Aging Produkte sind für die reifere Haut gut geeignet. Sie sind mit Wirkstoffen ausgestattet, die eine Hautalterung verzögern und das Bindegewebe stärken.
Die Konsistenz einer Anti Aging Creme ist besonders reichhaltig. Sie riecht frisch und zieht in den meisten Fällen ohne Rückstände ein. In den Anti Aging Cremes sind keine Silikone, Parabene oder PEGs vorhanden. Anti Aging Produkte sind für die reifere Haut gut geeignet. Sie sind mit Wirkstoffen ausgestattet, die eine Hautalterung verzögern und das Bindegewebe stärken.
Die Inhaltsstoffe sorgen dafür, dass die Haut immer elastisch bleibt. Besonders bei den Gesichtcremes werden Inhaltsstoffe wie Kollagen, Hyalouronsäure, Vitamin C und Coenzym Q10 eingesetzt, die Falten reduzieren und eine Hautalterung verlangsamen.
Ist die Wirkung von Anti Aging Produkten bewiesen?
Herkömmliche Anti Aging Cremes bringen häufig nicht immer den gewünschten Erfolg. Die Haut wirkt zwar durch die Inhaltsstoffe gepflegter, aber eine vermehrte Faltenbildung lässt sich dadurch nicht beseitigen. Neuartige pharmazeutische Cremes wirken dagegen wie ein pflanzliches Botox.
Ein großer Vorteil ist, dass sie nicht wie das Nervengift zu Muskelerschlaffungen oder den Verlust der persönlichen Mimik führen.
Können pharmazeutische Anti Aging Cremes Mittel wie Botox ersetzen?
Neben den bekannten Wirkstoffen und Fillern wie Retinol oder Hyaluron wurde durch Wissenschaftler jetzt der Anti-Falten-Wirkstoff Spilanthol entdeckt. Hierbei handelt es sich um ein Bio-Botox, welches aus Parakresse hergestellt wird. Es wirkt ähnlich wie ein Lokalästetikum.
Spilanthol wird schon seit vielen Jahren für die Behandlung von Zahn- oder Kopfschmerzen eingesetzt. Der Wirkstoff ist zwar nicht so stark wie das Nervengift Botox, hat aber auch nicht so starke Nebenwirkungen.
Wichtige Inhaltsstoffe, die in Anti Aging Cremes enthalten sein sollten
In pharmazeutischen Cremes gegen Hautalterungen sollte Alpha Liponsäure, Coenzym Q10, Hyaluronsäure, Vitamin A, Vitamin B3 und Vitamin C vorhanden sein. Durch das Zusammenspiel der Wirkstoffe wird die reifere Haut optimal gepflegt.
Durch den Zusatz von Vitamin A und pflanzlichen Ölen wird die Haut langanhaltend mit Feuchtigkeit versorgt. Während Nachtcremes häufig stärker fettend sind, können Anti Aging Cremes, die als Tagescreme genutzt werden, problemlos unter einem Make up aufgetragen werden.
Alpha Liponsäure: Die Alpha Liponsäure ist eine schwefelhaltige Fettsäure. Sie spielt beim Energiestoffwechsel eine große Rolle. Die Salze der Fettsäure werden auch als Lipoate bezeichnet. Der Inhaltsstoff erhöht den Gehalt an Coenzym Q10 und Vitamin C und wirkt zusätzlich antioxidativ.
Coenzym Q10: Coenzym Q10 sorgt für eine bessere Energieproduktion. Es ist vor allem für die Energie der Körperzellen zuständig. Selbst in den Mitochondrien, die als kleine Kraftwerke der menschlichen Zellen bezeichnet werden, sorgen sie für eine Erhöhung der Energie.
Hyaluronsäure: Hyaluronsäure wird zur Minderung von Falten eingesetzt. Der Stoff wirkt wie eine Art Wasserspeicher. Er sorgt dafür, dass die Haut mehr Elastizität erhält. Bis zu 6 Liter Wasser können mit einem 1 g Hyaluron gebunden werden. Aus diesem Grund wird sie in vielen Anti Aging Cremes eingesetzt.
Vitamin A (Retinol): Vitamin A ist besonders wichtig für die Funktion und zudem für den Aufbau und das Wachstum der Haut. Da das Vitamin nicht nur die Sehkraft stärkt, sondern auch für den Aufbau der gesunden Haut sorgt, ist es besonders wichtig. Durch Vitamin A werden die Zellen vor einer Oxidation geschützt.
Vitamin B3 (Niacin): Das Vitamin B3 gehört zu den Vitaminen aus dem Vitamin B-Komplex. Es wurde 1867 bei einer Oxidation von Nicotin entdeckt und ist ebenfalls für die Energiegewinnung sehr wichtig. Es wird in Anti Aging Cremes vor allem für die Unterstützung des Stoffwechsels eingesetzt und verschönert das Hautbild.
Vitamin C (L-Ascorbinsäure): Das Vitamin C hat eine antioxidative Wirkung und ist besonders gut für Pickel und Akne. Durch das Vitamin kann eine Mikroentzündung der Haut gelindert werden. Die entzündungshemmende Wirkung sorgt bei einer Anti Aging Creme für ein gesundes Hautbild. Durch den Einsatz des Wirkstoffs wird der Hautzustand deutlich verbessert.
Wie sinnvoll sind pharmazeutische Anti Aging Cremes wirklich?
 Eine pharmazeutische Anti Aging Creme ist auf jeden Fall sinnvoll. Die Inhaltsstoffe sind gut aufeinander abgestimmt. Die Haut wird bei einer regelmäßigen Anwendung gepflegt und mit ausreichend Feuchtigkeit versorgt. Feine Falten können bereits nach wenigen Wochen gelindert werden und das Hautbild wirkt elastischer. Einige Cremes bieten zusätzlich einen Schutz vor den schädlichen UV-Strahlen.
Eine pharmazeutische Anti Aging Creme ist auf jeden Fall sinnvoll. Die Inhaltsstoffe sind gut aufeinander abgestimmt. Die Haut wird bei einer regelmäßigen Anwendung gepflegt und mit ausreichend Feuchtigkeit versorgt. Feine Falten können bereits nach wenigen Wochen gelindert werden und das Hautbild wirkt elastischer. Einige Cremes bieten zusätzlich einen Schutz vor den schädlichen UV-Strahlen.
Wirksame Alternativen
Wirksame Alternativen zu Anti Aging Creme Produkten sind Cremes, die auf Hyaluron oder Meeresprodukten basiert. Wichtig ist, dass die Haut mit Feuchtigkeit versorgt wird und dabei nicht fettet. Für viele Nutzer ist es wichtig, dass die Anti Aging Alternativen aus natürlichen Inhaltsstoffen hergestellt werden, damit sie besser verträglich sind.
Fazit
Anti Aging Cremes sollten viele Mineralien, Hyaluron und Spilanthol enthalten. Dadurch wird gewährleistet, dass die Haut nicht nur gepflegter, sondern auch glatter aussieht. Feine Falten können somit geglättet werden und weiteren Falten vorgebeugt werden.
Weiterhin sollte darauf geachtet werden, dass die Creme kein Parfüm oder andere Inhaltsstoffe enthält, die die Haut reizen könnten. Je nach Hersteller stehen Anti Aging Cremes für tagsüber und nachts zur Verfügung. Nachtcremes geben der Haut mehr Feuchtigkeit, wodurch die Haut fettiger wirkt.
Tagescremes dagegen ziehen schnell ein und hinterlassen auf der Haut keinen unschönen Fettfilm.
Faltenunterspritzung – Diese pharmazeutischen Mittel gibt es
Es gibt heutzutage mehrere Möglichkeiten, Falten erfolgreich zu bekämpfen. Die Faltenunterspritzung wird immer beliebter, da die Ergebnisse dieser sich mit denen eines Facelift problemlos messen können. Zudem gibt es eine ganze Reihe an Vorteilen dieser Behandlungsmethode gegenüber den anderen gängigen Behandlungsmöglichkeiten. Bei der Unterspritzung kommen verschiedene pharmazeutische Mittel zum Einsatz.
Was ist eine Faltenunterspritzung?
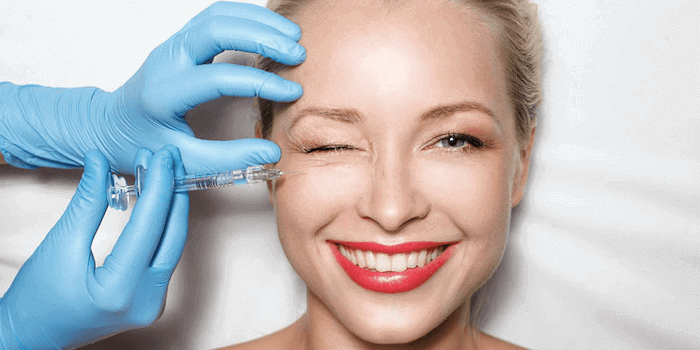 Unter der Unterspritzung wird eine Behandlungsmethode verstanden, bei der bestimmte pharmazeutische Mittel unter die Haut gespritzt werden, um die Falten im Gesicht zu reduzieren. Sie stellt eine sinnvolle Alternative zum Facelift dar, da es sich um eine nicht-operative Maßnahme handelt.
Unter der Unterspritzung wird eine Behandlungsmethode verstanden, bei der bestimmte pharmazeutische Mittel unter die Haut gespritzt werden, um die Falten im Gesicht zu reduzieren. Sie stellt eine sinnvolle Alternative zum Facelift dar, da es sich um eine nicht-operative Maßnahme handelt.
Sie ist relativ schmerzlos und mit kaum Komplikationen verbunden. Die häufgsten Behandlungsregionen, für welche eine Unterspritzung angeraten wird, sind die Nasen-, Mund- und Stirnfalten. Das untergespritzte pharmazeutische Mittel hebt die Falten von unten und glättet sie.
Welche Arten von pharmazeutischen Mitteln gibt es?
Bei der Unterspritzung kommen verschiedene pharmazeutische Mittel, wie Botox oder unterschiedliche Dermal-Filler, zum Einsatz. Diese unterscheiden sich voneinander in der Art und Zusammensetzung und dienen unterschiedlichen Zwecken.
Welche pharmazeutische Mittel letztlich zum Einsatz kommen, wird vor der Behandlung in einem ausführlichen Beratungsgespräch mit dem Arzt geklärt.
Botulinomtoxin (Botox)
Botulinomtoxin, auch Botox genannt, wird von verschiedenen Bakterienstämmen der Gattung Chlostridium gebildet. Es handelt sich um einen natürlich vorkommenden Eiweißstoff, welcher in der Medizin schon länger bei der Behandlung von neurologischen Bewegungsstörungen zum Einsatz kommt.
Seit den 90-er Jahren wird das pharmazeutische Mittel stark verdünnt zur Unterspritzung verwendet und gilt als ein gut verträgliches Präparat. Jedoch nur unter der Voraussetzung, dass es von einer Fachperson angewendet wird, da eine falsche Anwendung zu Komplikationen führen kann.
Dermal-Filler
Neben Botox kommen bei der Unterspritzung auch die sogenannten Dermal-Filler zum Einsatz. Es handelt sich dabei um pharmazeutische Mittel, welche über einen Zeitraum von mehreren Monaten vom Köper rückstandslos ausgeschieden werden. Dies liegt an der Tatsache, dass es sich bei den Dermal-Fillern um Stoffe handelt, welche natürlich im Bindegewebe der Haut vorkommen.
Dies bedeutet zugleich, dass ihre Anwendung mit geringen Risiken verbunden ist, sodass sie bei der Faltenbehandlung sehr beliebt sind.
Hyaluronsäure: Die Hyaluronsäure ist ein körpereigener Stoff und besitzt ein hohes Wasserbindungsvermögen, sodass diese für die Aufrechterhaltung der Spannkraft und der Elastizität der Haut verantwortlich ist. Der Gehalt der im Bindegewebe der Haut befindlichen Hyaluronsäure nimmt im Alter ab, sodass es zu Falten kommt.
Durch die Unterspritzung mit Hyaluronsäure gewinnt die Haut erneut an Spannkraft und Elastizität. Sie kann sowohl punktuell, als auch großflächig angewendet werden.
Kollagen: Kollagen stellt ebenfalls einen festen Bestandteil der Haut dar, welcher für die Festigkeit der Haut verantwortlich ist. Das bei der Unterspritzung verwendete Kollagen wird aus Rindergewebe gewonnen und steht in verschiedenen Formen zur Verfügung.
Dieses pharmazeutische Mittel sorgt dafür, dass sowohl oberflächliche als auch tiefere Falten effektiv geglättet werden und das Volumen insgesamt sichtlich verbessert wird.
Eigenfett: Die Behandlung mit Eigenfett wird besonders Allergikern empfohlen. Denn zu diesem Zweck entnimmt der Arzt mit speziellen Instrumenten frische, lebende körpereigene Fettzellen an geeigneter Körperstelle. Die Entnahme selbst ist schmerzlos.
Nach der sorgfältigen Aufbereitung der Fettzellen werden diese zur Unterspritzung verwendet.
Polymilchsäure: Die Unterspritzung mit Polymilchsäure, einer synthetisch hergestellten Substanz auf Basis der körpereigenen Milchsäure, ist relativ neu. Diese verbessert nicht nur das Volumen, sondern regt auch die Bildung von körpereigenem Kollagen an.
Sie kommt bei tieferen Falten zum Einsatz, kann aber auch bei oberflächlichen Falten angewendet werden.
Polycaprolacton: Polycaprolacton gehört ebenfalls zu neueren Dermal-Fillern. Hierbei handelt es sich um einen biologisch abbaubaren Stoff, welcher schon seit mehreren Jahrzehnten in der Medizin bei der Wundheilung zum Einsatz kommt. Polycaprolacton regt die körpereigene Kollagenproduktion an und weist eine Wirkungsdauer von etwa 12 bis 36 Monaten nach der Unterspritzung auf.
Calcium Hydroxylapatit: Calcium Hydroxylapatit ist ein weiterer biologisch abbaubarer Stoff, welcher zur Unterspritzung mit einem Trägergel aus Glycerin, Wasser und Carboxymethylcellulose zum Einsatz kommt. Dadurch weist er einen doppelten Wirkmechanismus auf.
Einerseits wird die Kollagenbildung angeregt und andererseits sind dank der Gel-Matrix die Ergebnisse sofort sichtbar.
Welche Komplikationen kann es bei einer Faltenunterspritzung geben?
 Die Unterspritzung verläuft meist ohne Komplikationen. Doch diese können bei unsachgemäßer Anwendung auftreten. Bei falsch durchgeführten Injektionen oder bei Überdosierung der pharmazeutischen Mittel können Schwellungen auftreten, welche sogar länger anhalten.
Die Unterspritzung verläuft meist ohne Komplikationen. Doch diese können bei unsachgemäßer Anwendung auftreten. Bei falsch durchgeführten Injektionen oder bei Überdosierung der pharmazeutischen Mittel können Schwellungen auftreten, welche sogar länger anhalten.
Die falsche Anwendung von Botox kann sogar zu Lähmungserscheinungen führen. Wird die Unterspritzung sachgemäß durchgeführt, ist die Behandlungsmethode relativ bedenklos.
Fazit
Die Unterspritzung gilt als eine gute Alternative zum Facelift. Denn es handelt sich hierbei um eine Behandlungsmethode welche stationär durchgeführt wird und nahezu schmerzlos ist. Während der Behandlungsmethode werden mithilfe von dünnen Nadeln verschiedene pharmazeutische Mittel in die betroffene Hautregion gespritzt.
Es handelt sich bei diesen um Stoffe, welche im Bindegewebe der Haut natürlich vorkommen, sodass sie nach einiger Zeit vom Körper verstoffwechselt werden. Mit dieser Faltenbehandlungsmethode lassen sich sowohl oberflächliche als auch tiefere Falten effektiv glätten.
In Abhängigkeit davon, welche pharmazeutischen Mittel dabei zum Einsatz kommen, halten die Ergebnisse mehrere Monate oder sogar Jahre an. Die Unterspritzung gilt als eine sichere Behandlungsmethode, welche mit geringen Risiken verbunden ist. Doch dies ist nur dann der Fall, wenn diese auch sachgemäß durchgeführt wird.
Aus diesem Grund sollte man sich diese ausschließlich von einem Facharzt durchführen lassen. Denn dieser sorgt sowohl dafür, dass optimale Ergebnisse durch die Unterspritzung erreicht werden, als auch dafür, dass passende pharmazeutische Mittel ausgewählt werden.





 Definition von Best Practices – Wir zeigen welche Produkte unseren Test standhalten konnten.
Definition von Best Practices – Wir zeigen welche Produkte unseren Test standhalten konnten.